Carbon Disulfide Commonly Tested Lewis Structures. To draw the CS2 lewis structure we have to find out the CS2 valence electrons firstWe express valence electrons as dots in CS2 lewis dot structure.

Gambarkan Struktur Lewis Dari Senyawa Berikut Diketahui 0 8 F 9 S 16 C 6 A Of2 B So3 C Cs2 D Brainly Co Id
A sulphur atom must be the central atom for the structure to be stable.

Struktur lewis cs2. Can anyone try it. Aprende a realizar las estructuras de Lewis tomando como ejemplo el compuesto CS2. B What are the approximate bond angles.
To get the valence electrons of carbonwe need to look at the electronic configuration of carbon. A step-by-step explanation of how to write the Lewis Dot Structure for CS2 Carbon DiSulfide. Join Login 11th Chemistry Chemical Bonding and Molecular Structure.
This species has its three atoms bonded sequentially in the following fashion. What is the Lewis dot structure for CS. Find the center atom of this compound.
Draw the bonding scheme and state sigma and pi bonds. For the CS2 Lewis structure calculate the total number of valence electrons for the CS2 molecule. S C S.
Carbon is the least electronegative atom and goes in the center of this structure. Expert AnswerGet homework answers from experts in Math Physics Programming Chemistry Economics Biology and more. Lewis framework for CS2.
Our experts will help you understand the concepts formulas to apply and solve homework problems for achieving better grades in class. Sigue los pasos y resuelve cualquier estructuraNo olvides suscri. To answer this problem we must.
B The rightmost bond between C and S is a triple bond. Cs2 Lewis Structure Drawing How to Draw the Lewis Dot Structures of Cl2 and CS2 YouTube Ch 4 lecture 4 StepsCS2 Lewis Structure Lewis Structure for CS2 ICl4 Lewis Structure How to Draw the Lewis Structure. There are 16 valence electrons for the CS2 Lewis structure.
The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in order to fill the octet of Carbon. It depends on the octet rule concept and is an extension of the electron dot diagram. After determining how many valence electrons there are in CS2 place them around the central atom to complete the octets.
Draw the Lewis structure for CS2 a What is the electron pair geometry and molecular geometry. Can anyone try it. The lewis dot structure for CS2 also predicts many of the properties of the molecule.
Draw up and down each S. This page can help you. -the reactivity of a molecule and how it might interact with other molecules.
Were being asked to draw a Lewis structure for CS 2. This is done by taking. Get 247 cs2 lewis structure assistance at TutorEye.
-the shape of a molecule. C Draw the 3D structure and use polarity arrows to show whether the compound is polar or nonpolar. Cs2 Lewis Structure What is the Lewis structure of CS2.
The geometry of the CS2 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the CS2 geometrical shape in which the electrons have from one another. SCS Drawing Picture on each one. Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure.
Write the Lewis structure for carbon disulfide CS2. SamarExpert Tutor Step 1 of 3 To draw the Lewis structure of we need to calculate the total number of valence electrons. The CS2 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CS2 molecule.
Due to the presence of large sulfide atoms over for comparison oxygen atoms in CO2 the molecule has a greater capacity for temporary london dispersion forces. Full load 0. Carbon 4 connections 44 0.
Thus to have a comprehensive idea about CS2 Lewis Structure let us go through each step clearly and systematically. Click hereto get an answer to your question Which of the following statement is true about the most stable Lewis structure for CS2. We draw Lewis Structures to predict.
Lewis Structure for CS 2 Carbon Disulfide Lewis Structure for CS. C61s² 2s²2p² The highest value of principal quantum number here is n2. In the most important resonance structure of CS2.
Does CS2 have resonance structures. What is the Lewis structure of CS2. A The leftmost bond between S and C is a single bond.
-the physical properties of a molecule such as boiling point surface tension etc. Get the best online tutoring services for K-12 and College students from highly qualified tutors at TutorEye. S 4 electrons 2 bonds 66 0.
As a result there can be more induced dipoles which increases the solubility of CS2. D What is the hybridization of the central atom. Draw the Lewis dot structure for CS2.
Xs are C-valence. Determine the total valence electrons present.

Gambarlah Rumus Lewis Dan Rumus Bangun Utk Molekul Berikut A Cs2 B Hcn Brainly Co Id

Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube

How To Draw The Lewis Structure Of Cs2 Carbon Disulfide Youtube

Tentukan Jenis Ikatan Yg Terdapat Dalam Senyawa Berikut Struktur Lewis A Cs2 B Of2 C Brainly Co Id

The Most Stable Lewis Structure Of N2o Is

Gambar Rumus Lewis Dalam Moleku Molekul A Cs2 B H2s C Nh3 D Hcn E H2o Brainly Co Id

Gambar Lah Rumus Lewis Dan Rumus Bangun Molekul A Cs2 B Hcn Brainly Co Id
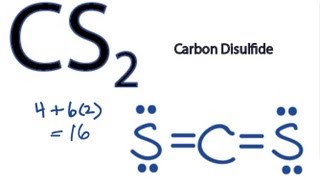
Lewis Structure For Cs2 Carbon Disulfide
Comments
Post a Comment